Mechanobiology of Blood: from Blood Clotting to Tissue Regeneration
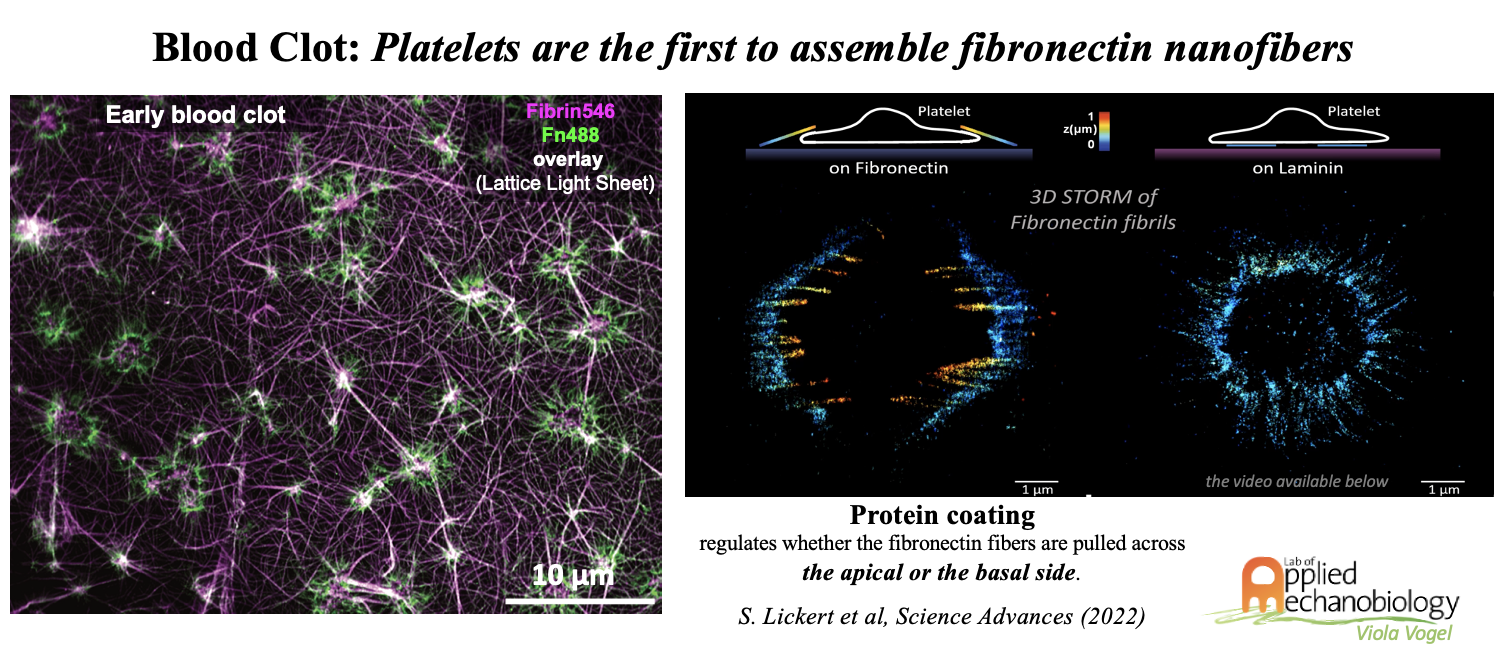
Blood clotting to stop the bleeding at sites of injury is essential for survival, yet if activated by other factors, microthrombi can form and be released into the blood circulation with sometimes lethal consequences when plugging up capillaries. Blood coagulation is a multistep process involved in the development of different physiological and pathological conditions such as wound healing and bleeding associated disorders. Platelets are the key players to limit blood loss at the site of vascular injury by forming a hemostatic plug in a coordinated process. Blood coagulation is initiated at sites of injury once platelets get activated by exposure to the basement membrane proteins of damaged blood vessels. While the biochemistry of blood coagulation is well established, far less is known how the mechanobiology of platelets steers the remodeling of blood clots and thus define the first steps of tissue repair. Platelets not only secrete the biochemical building blocks necessary to rapidly assemble fibrin rich thrombi, but that they also start to contract the wound site and we could show that platelets assemble the very first fibronectin fibers, and not the invading cells.
The mechanobiology of single platelets and their ability to assemble fibronectin fibers was thereby probed by super-resolution microscopy (STORM) and with micropillar assays (Sebastian’s paper). Platelets thereby play a crucial first role in the initiation of tissue regeneration processes after injury and if dysregulated, they tip the balance toward fibrotic pathologies. To investigate the mechanobiology of platelets and blood coagulation processes, we use state-of-the-art microscopy in combination with state-of-art bioengineering and molecular biology techniques to characterize the adhesion machinery of platelets during spreading in different physical and biochemical environments, and to understand how platelets react to different coagulation cascade factors and trigger the molecular pathways underlying the mechano-mediated modulation of blood clot formation. Our research revealed furthermore that blood cells entrapped in a blood clot in synergy with invading fibroblasts promote the creation of a pro-angiogenic microenvironment during early phases of wound healing (Melanie Burkhard’s papers).
Key papers:
- external pageBurkhardt, M., Waser, J., Milleret, V, ... & Vogel V. et al. (2016). Synergistic interactions of blood-borne immune cells, fibroblasts and extracellular matrix drive repair in an in vitro peri-implant wound healing model. Sci Rep 6, 21071call_made
- external pageLickert, S., Kenny, M., Selcuk, K., Mehl, J. L., Bender, M., Früh, S. M., ... & Vogel, V. (2022). Platelets drive fibronectin fibrillogenesis using integrin αIIbβ3. Science Advances, 8(10), eabj8331.call_made