Mechanobiology of Immune Cells in Health and Disease
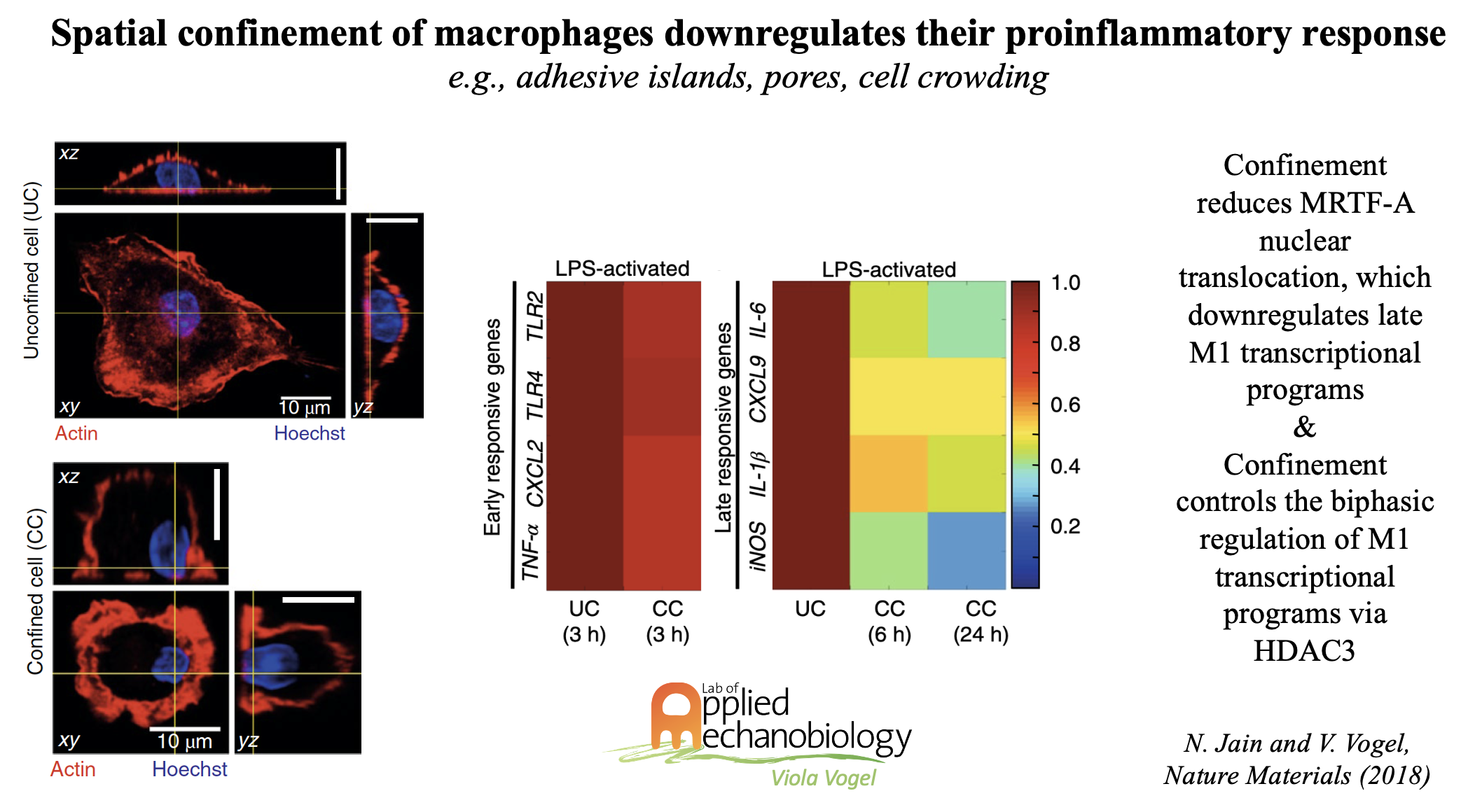
While much of what is known today about the functional regulation of immune cells has been derived from 2D cell culture dishes, immune cells in their native environments either circulate in blood, or are found in highly complex and organ-specific cell niches, often densely packed with other cells or exposed to porous environments. As immune cells are typically not free to spread in their native tissue niches, we thus explored how different modalities of spatial confinement might impact their function. Using micro- and nanoengineered substrates, we discovered that special confinement significantly downregulates the proinflammatory response of LPS-stimulated macrophages. This is significant as macrophages are activated upon injury or the onset of a variety of inflammatory diseases into a spectrum of states, from pro-inflammatory (M1) to pro-healing (M2) phenotypes, with gradually altered transcriptional profiles and functional outputs. Macrophages respond to chemical/metabolic and physical stimuli, but their effects cannot be readily decoupled in vivo during proinflammatory activation. We could show that preventing macrophage spreading by spatial confinement, as imposed by micropatterning, microporous substrates or cell crowding, suppresses several transcriptional programs by mechanomodulating chromatin compaction and epigenetic alterations.
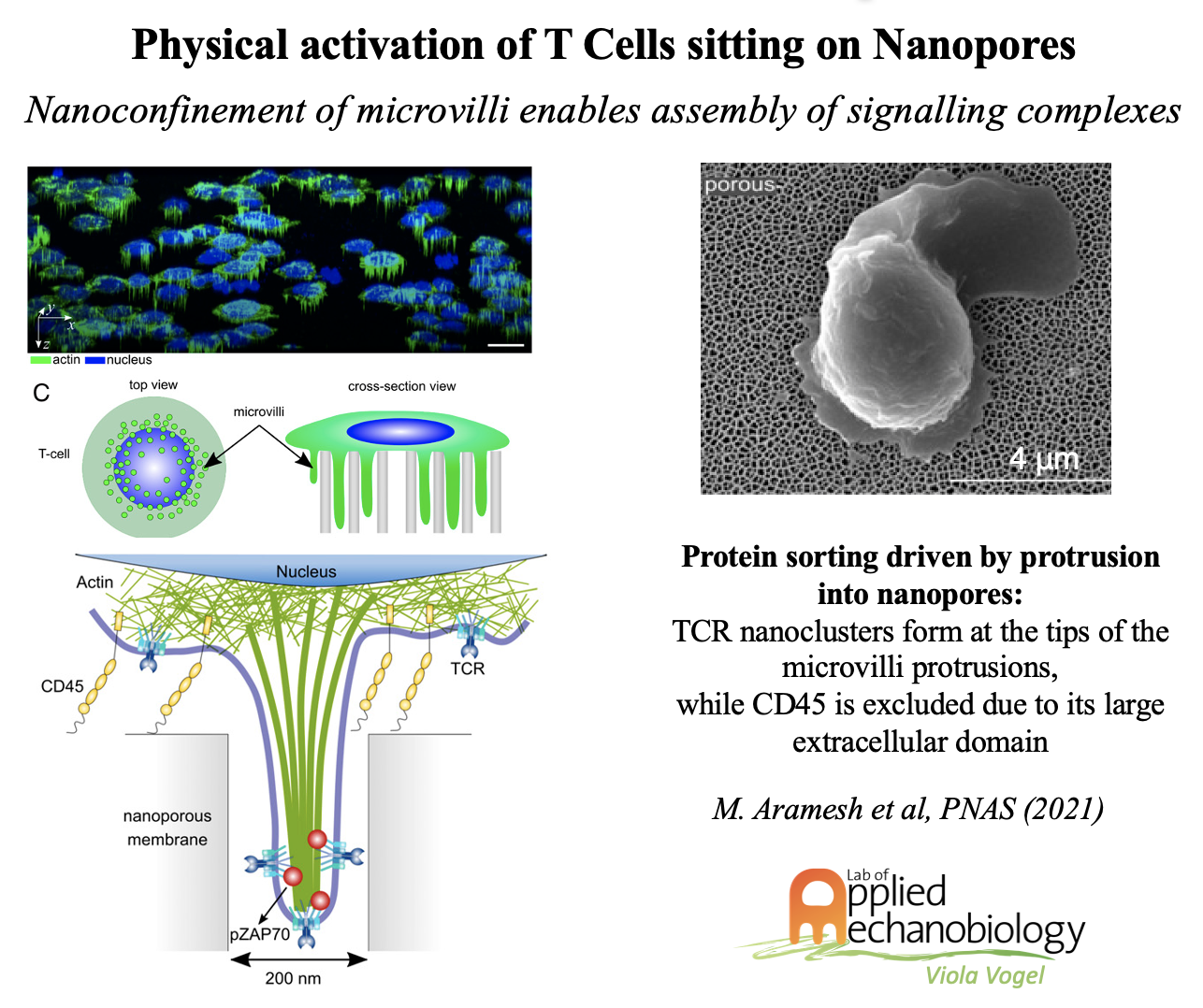
In contrast to macrophages, T cells are the cornerstone of adaptive immune response, and the development of new approaches to tailor and prime T cells is crucial to improve on the efficacy of adoptive immunotherapies. Throughout their lifetime and in their native environments, T cells are constantly exposed physical constraints and mechanical forces. Whereas the concept of antigen-based stimulation is well established, how physical stimuli modulate T cell activation and T cell fate is largely unknown. In collaboration with the Klotzsch group (HU Berlin, Germany), we could recently show the importance of traction forces that T cells apply via their actin-rich microvilli. In our most recent publication, we discovered how physical constraints of nanoporous substrates can activate T cells independent of any biochemical stimuli. Finally, we also discovered recently, that mouse-grown tumors are rich in track structures, presumably originating from transformed blood vessels, that harbor cancer cells together with CD8+ cells and M2 macrophages, embedded in an ECM rich in collagen and relaxed fibronectin fibers which together create an immune-suppressive environment.
Key papers:
- Jain, N., & Vogel, V. (2018). Spatial confinement downsizes the inflammatory response of macrophages. Nature materials, 17(12), 1134-1144.
- Mehl, J. L., Earle, A., Lammerding, J., Mhlanga, M., Vogel, V., & Jain, N. (2022). Blockage of lamin-A/C loss diminishes the pro-inflammatory macrophage response. Iscience, 25(12), 105528.
- Fonta, C. M., Loustau, T., Li, C., Surendran, S. P., Hansen, U., Murdamoothoo, D., Benn M. C., Velazquez-Quesada I., Carapito R., Orend G. & Vogel, V. (2023). Infiltrating CD8+ T cells and M2 macrophages are retained in tumor matrix tracks enriched in low tension fibronectin fibers. Matrix Biology, 116, 1-27.