Mechanobiology: from Cells to Tissue- Mimetic Multicellular Niches
Organ-specific cell niches are not composed of cell monocultures, as conventionally studied in biology, but of a large variety of cell types which are in close contact to each other and communicate via biochemical and physical factors. Much progress has been made in the molecular understanding of how physical stimuli are sensed and transduced by cells into biochemical signals (mechano-signaling and mechano-transduction), which then regulate gene transcription processes and subsequently cell decision making. Beyond the physical properties of cellular microenvironments, that engineers could easily produce and tune, the reciprocal mechanical signaling between cells and their three-dimensional (3D) extracellular environments greatly impacts cell decision making.
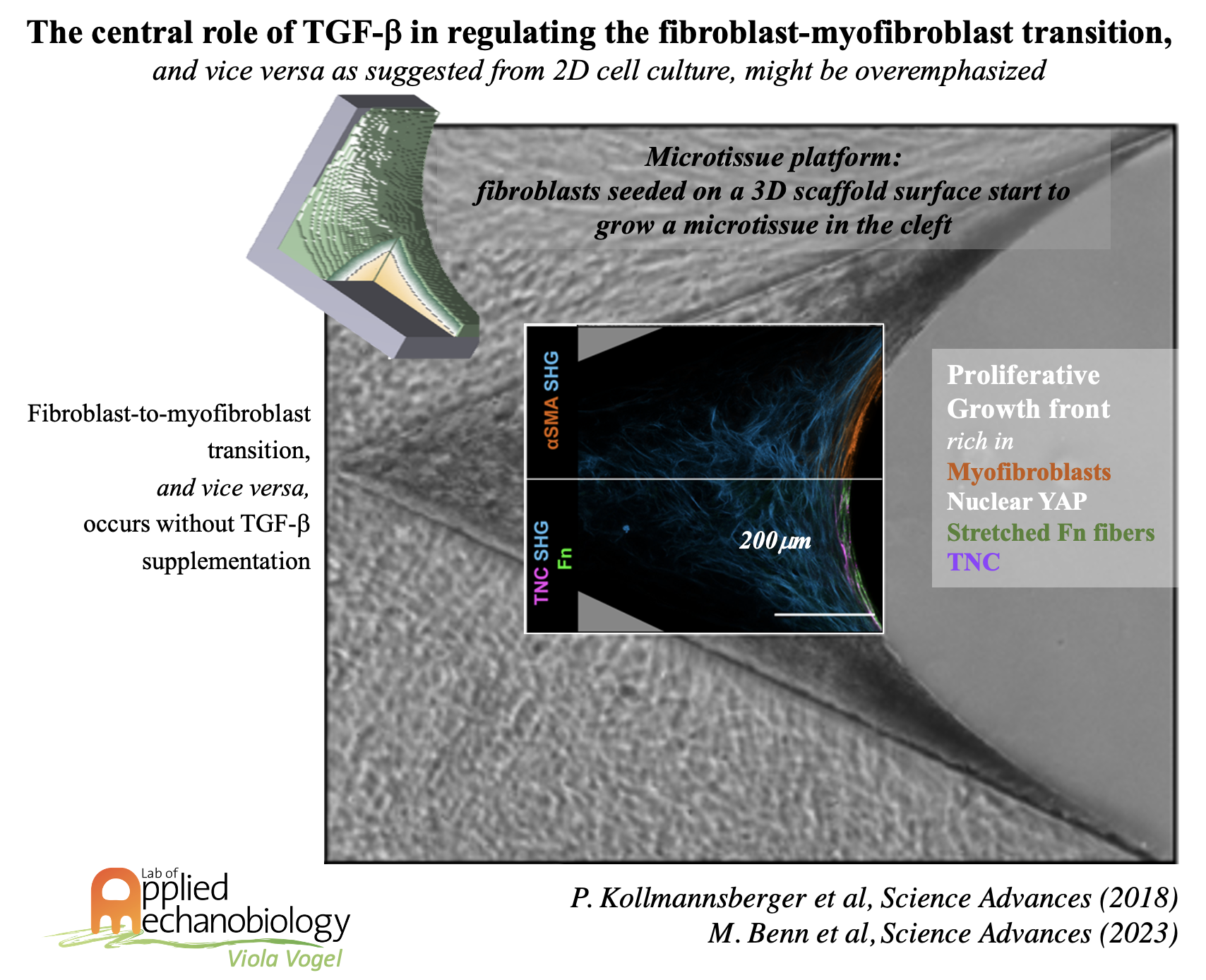
While much mechanobiology has been learned at the cellular level, far less is known how these insights can be translated to the tissue level. Microtissue platforms thereby play a major role to study time-dependent tissue formation and remodeling processes. We are thus creating well controlled three-dimensional microtissues to mimic healthy versus pathologically transformed tissues. To test translational hypotheses, we are using de novo grown or cell-seeded tissue engineered scaffolds. This includes the creation of an intimal hyperplasia cell sandwich system and a heart valve on the dish model, as well as de-novo grown microtissues made from fibroblasts which allowed us to show that tensile forces drive a reversible fibroblast-to-myofibroblast transition during de novo 3D tissue growth in engineered clefts, even upon TGFb inhibition. A more refined microtissue platform now revealed details about the reciprocal crosstalk of fibroblasts with their newly assembled ECM, and the role of forces in regulating the crosstalk.
Also, bone marrow provides a fantastically complex environment in which bone can form and be resorbed in one compartment, which encloses a highly vascularized compartment in which blood and all of our immune cells are produced by the differentiation of hematopoietic stem and progenitor cells (HSPCs). As blood can currently not yet be produced in bioreactors in vitro, our current goal is to create a bone marrow-mimetic niche system. These new in-vitro systems thus offer new tools to study basic mechanisms of cell-ECM communication, while providing an easy-to-use translational disease model platforms at low-cost for drug screening and development.
Key papers:
- Kollmannsberger, P., Bidan, C. M., Dunlop, J. W., Fratzl, P., & Vogel, V. (2018). Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Science advances, 4(1), eaao4881.
- Hosseini, K., Taubenberger, A., Werner, C., & Fischer‐Friedrich, E. (2020). EMT‐induced cell‐mechanical changes enhance mitotic rounding strength. Advanced Science, 7(19), 2001276.
- Fernandes, A., Miéville, A., Grob, F., Yamashita, T., Mehl, J., Hosseini, V., ... & Vogel, V. (2022). Endothelial‐Smooth Muscle Cell Interactions in a Shear‐Exposed Intimal Hyperplasia on‐a‐Dish Model to Evaluate Therapeutic Strategies. Advanced Science, 9(28), 2202317.