Dr. Enrico Klotzsch
Dr. Enrico Klotzsch
Staff of Lehre HEST
Experimental Biophysics

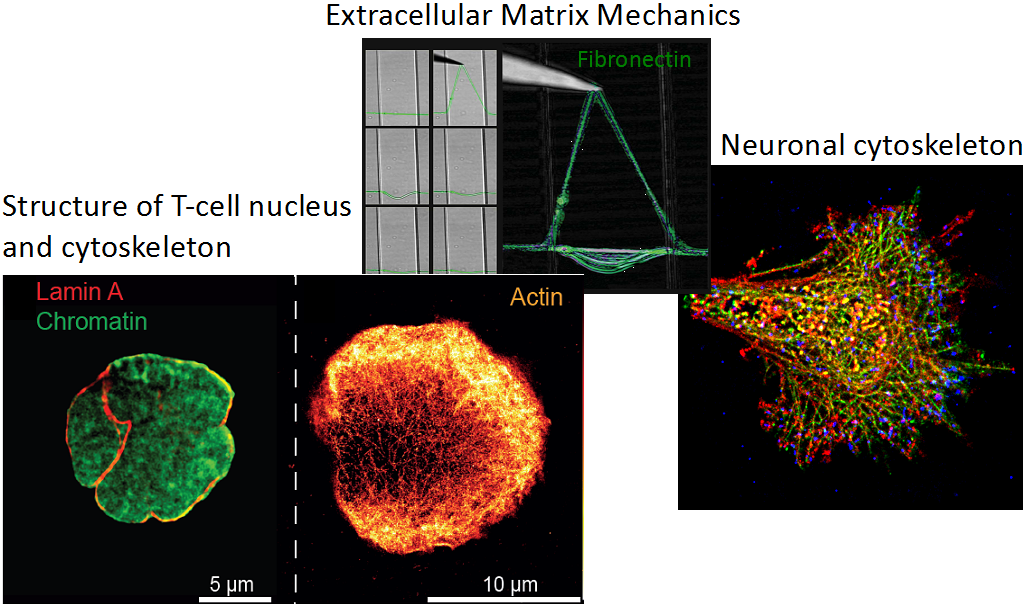
Structure - not only on the molecular level – defines function. Our group aims in bridging the gap between structural and cell biology, particularly to decipher the mechanical aspects of how cells sense and react to their environment. This includes asking how cells employ forces to probe the interaction to other molecules such as receptor-ligand interactions to switch biochemical function, i.e. T-cell activation, migration or their interaction with the extracellular matrix. Furthermore, we investigate how these insights can be exploited for biomedical applications.
We use state-of-the-art single molecule microscopy techniques such as super-resolution microscopy, AFM and optical tweezer to gain insights into general aspects of mechanobiology, membrane biophysics, neurobiology and immunology.
Website: external pagewww.biologie.hu-berlin.de/de/gruppen/jpexpbp_portal/call_made
Papers:
1. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. E Klotzsch, ML Smith, KE Kubow, S Muntwyler, WC Little, F Beyeler, D Gourdon, BJ Nelson, V Vogel (2009). Proceedings of the National Academy of Sciences 106 (43), 18267-18272
2. Superresolution microscopy reveals spatial separation of UCP4 and F0F1-ATP synthase in neuronal mitochondria. E Klotzsch, A Smorodchenko, L Löfler, R Moldzio, E Parkinson, GJ Schütz, EE Pohl (2015). Proceedings of the National Academy of Sciences 112 (1), 130-135
Contribution to research networks
Australian Research Council - Defining the spatial and temporal regulation of neurite branching
The project is in collaboration with Prof. Dr. T. Fath at UNSW Sydney. The overarching aim of this project is to use super-resolution microscopy to decipher the role of the actin cytoskeleton-associated protein tropomyosin in cell extension of nerve cells (neurons) which is essential for establishing neuronal networks in vitro and in vivo.

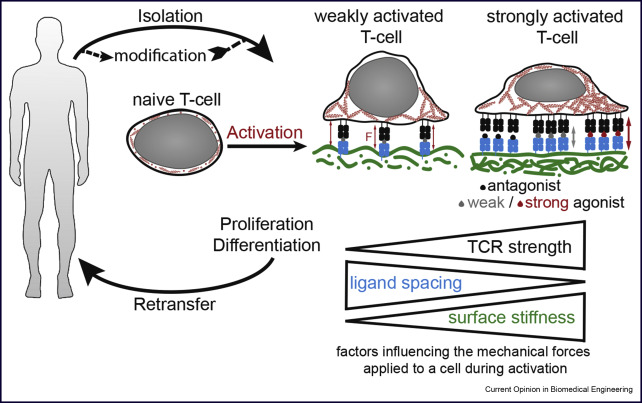
HFSP - Mechanical regulated gene expression during T-cell activation.
Dr. Klotzsch together with Dr. Ries (EMBL Heidelberg) got awarded the Human Frontiers Science Program Young Investigator Award this project will be carried out at ETH Zurich, Switzerland, where Dr. Klotzsch holds a second affiliation. The spatial organization of chromatin is of key importance for gene expression and maintenance. Different from stem cell differentiation, during T-cell activation gene regulation happens on a much faster timescale, as the immune response is critical for the bodies defense mechanism. A likely regulatory mechanism for this is the chromatins’ 3D structure and its interaction with nuclear lamina.
Here we will study how mechanical forces `ultimately regulate gene expression. We hypothesize that mechanical force triggers structural/conformational changes of nuclear lamina network; hence genes encoded in chromatin proximal to lamina can be up/downregulated. We propose to develop new Super-Resolution Microscopy and live-cell imaging technologies and combine them with single cell transcriptomic and epigenetic markers in a correlative approach to resolve the 3D structure of lamina associated chromatin domains and their dynamics during T-cell activation.
The Latest Publication
Engineering T-cell Activation for Immunotherapy by Mechanical Forces
M. Aramesh, D. Stoycheva, L. Raaz and E. Klotzsch
Current Opinion in Biomedical Engineering, 2019. external pageLinkcall_made (Open Access)
Are you interested?
We are currently looking for talented Master and Bachelor Students in the field of T-cell Mechanobiology. Our lab specializes in fluorescence microscopy including Förster resonance energy transfer (FRET), super-resolution imaging (STORM, PALM), AFM and lattice light sheet microscopy. In addition to advanced microscopy, we offer training in biochemical, molecular biological and cell biological approaches.
Please send through your CV and one-page motivation for application to or
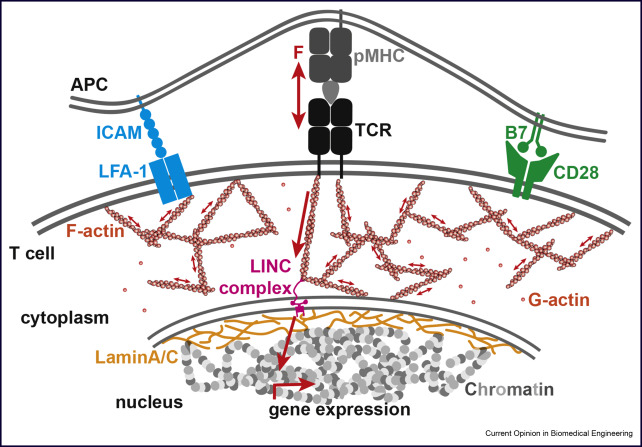
Forces in T-cell activation
T-cells readily detect the presence of even a single antigenic peptide-major histocompatibility complexes (pMHC) complex and discriminate among thousands of endogenous pMHC via T-cell receptors (TCRs) on the surface of antigen presenting cells. The mechanisms underlying this phenomenal sensitivity have remained elusive. Recent studies suggest mechanical forces to be instrumental in antigen sensing and discrimination. To address the role of forces and the origin of forces, we employ Traction Force Microscopy (TFM) into the immune synapse that allow us to quantitatively visualize forces acting between TCR and pMHC on opposing cell surfaces. Furthermore, we employ super-resolution microscopy to study simultaneous cytoskeletal remodeling. In summary, the projects aims to understand, how mechanical forces orchestrates receptor-ligand interactions during immune synapse formation and T cell activation.
Supervisors: Dr. Morteza Aramesh, Prof. Dr. Enrico Klotzsch, Prof. Dr. Viola Vogel
Altering gene expression through force induced nuclear rearrangements
The overall aim of this proposed research is to establish optical super-resolution microscopy as a technique to connect mechanobiological aspects of T-cell activation with altered gene expression. By combining cutting-edge imaging, mechanobiological and genetic tools, we will be able to answer the open question whether T cells use mechanical forces to distinguish between pathogens and furthermore, how these forces are transmitted into the nucleus to alter gene expression. This will be the first time a direct link between an extracellular cue and genetic regulation will be demonstrated and we will be able to dissect the mechanism.
Supervisors: Dr. Morteza Aramesh, Prof. Dr. Enrico Klotzsch and Prof. Dr. Viola Vogel
